Sickle-Cell Treatment Created With Gene Editing Wins U.K. Approval
Regulators in Britain on Thursday approved the first treatment derived from CRISPR, the revolutionary gene-editing method. Called Casgevy, the treatment is intended to cure sickle-cell disease and a related condition, beta thalassemia.
The manufacturers, Vertex Pharmaceuticals, based in Boston, and CRISPR Therapeutics, based in Switzerland, say about 2,000 patients in Britain with sickle-cell disease or beta thalassemia are expected to be eligible for its treatment.
The companies anticipate that the Food and Drug Administration will approve Casgevy for sickle-cell patients in the United States in early December. The agency will decide on approval for beta thalassemia next year.
In late December, the F.D.A. is expected to approve another sickle cell gene therapy by Bluebird Bio of Somerville, Mass. That treatment does not rely on gene editing, insteading using a method that inserts new DNA into the genome.
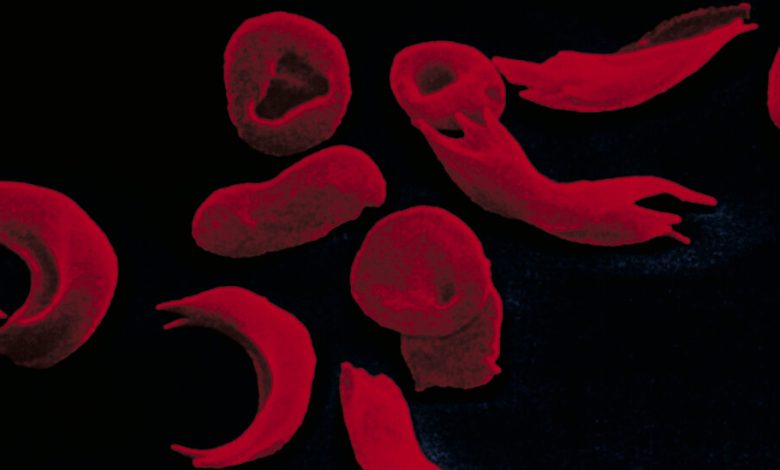
Sickle-cell disease is caused by a defective gene that leads to the creation of abnormal hemoglobin, the oxygen-carrying component in red blood cells. The cells themselves become malformed, causing episodes of extreme pain. About 100,000 Americans, who are mostly Black and Hispanic, are believed to have the illness.
In beta thalassemia, the defective gene leads to deficient levels of hemoglobin in red blood cells. The condition is rare.
Casgevy relies on CRISPR to nick the DNA, activating a gene that produces an alternative form of hemoglobin. To receive the sickle-cell treatment, patients in Britain must be at least 12 years old and have experienced repeated episodes of extreme pain.
There is no upper age limit, nor are patients excluded because they have suffered too much organ damage from sickle-cell disease, said Dr. David Altshuler, Vertex’s chief scientific officer.
But the patients must have no other options. Sickle-cell disease can be cured with a bone-marrow transplant, but few patients have compatible donors.
For people struggling with the illness, the Vertex and Bluebird treatments have been a long time coming. Pain is not the only complication — people with sickle-cell disease also suffer bone and organ damage and strokes. The misshapen blood cells do not survive long, resulting in anemia.
Still, the CRISPR and Bluebird treatments are onerous and will require expertise that most hospitals lack.
Patients must receive intense chemotherapy to clear their bone marrow of abnormal stem cells and make room for the genetically altered cells. Then the patients must stay a month or more in a hospital while their marrow regrows.
And gene editing is expensive. Vertex and CRISPR Therapeutics have not set a price yet in Britain — that will depend on conversations with those who will be paying for it, said Stuart Arbuckle, executive vice president and chief operating officer at Vertex.
The price in the United States, though, is expected to be millions of dollars per patient. Sickle-cell disease itself is expensive, however, costing the U.S. health system an estimated $3 billion a year.
In the United States, Bluebird already has a gene therapy approved for beta thalassemia. It costs $2.8 million per patient.
Dr. Altshuler said Vertex was testing its sickle-cell treatment in children ages 5 to 11, hoping to prevent the irreversible organ damage that occurs over time.
The company’s first sickle-cell patient, Victoria Gray, said on Thursday that the treatment changed her life.
Ms. Gray, a Walmart associate in Forest, Miss., was diagnosed with sickle-cell disease when she was 3 months old and had a pain crisis. Those episodes became a part of her life, resulting in frequent hospitalizations.
“A lot of my dreams, I couldn’t do,” she said. “The smallest things — cold, changing weather — I would end up in the hospital.”
She had the gene editing treatment in 2019, when she was 33. Now, she said, all her symptoms have vanished.
“It meant a new beginning,” Ms. Gray said. “It is more than I ever dreamed of, for everything to be gone.”




